China drug master file (DMF) policy was implemented by China NMPA (formerly CFDA) in July 2019. Under this policy, it is common for drug product applicants to expect their suppliers to obtain China DMF number(s) prior to drug submission, ensuring confidential data review by China CDE without disclosing information to the drug client.
Scope of China DMF
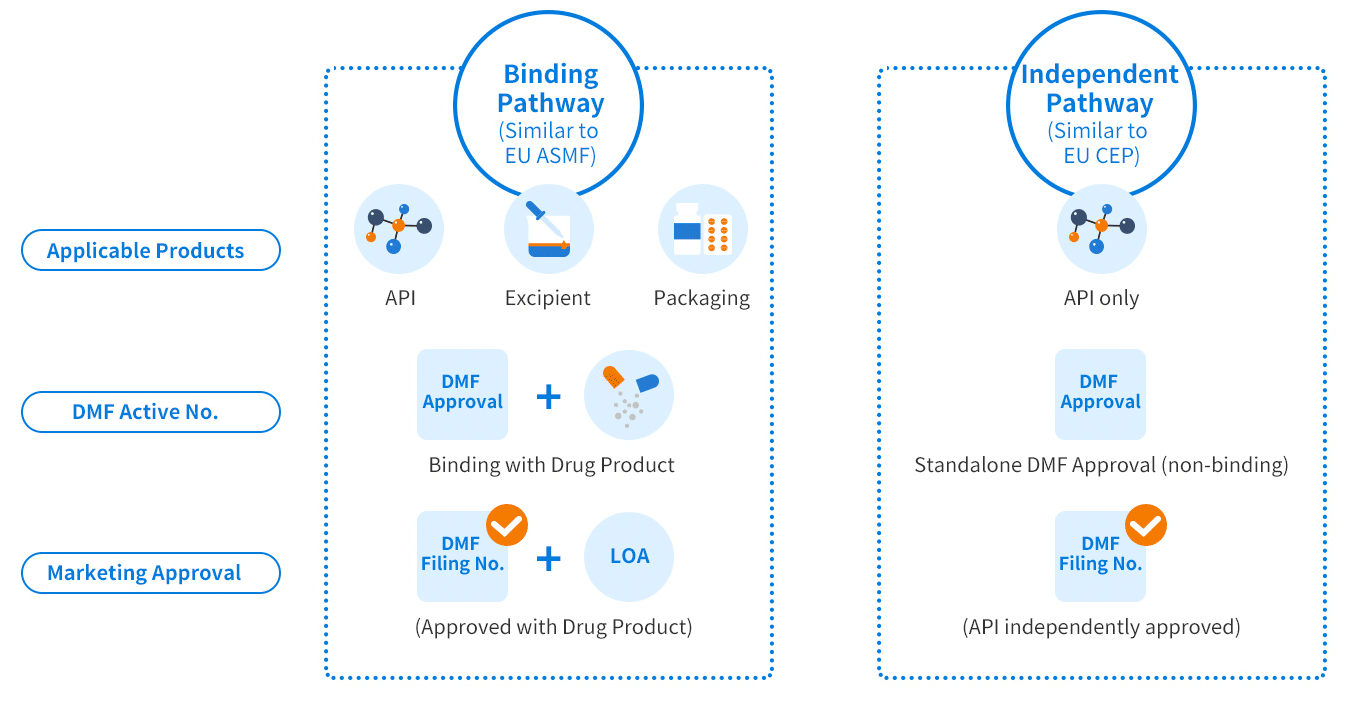
China DMF covers three categories:
- Active pharmaceutical ingredients (APIs)
- Excipients
- Packaging materials
Overlaps in China DMF and US DMF
The China DMF regulatory system shares similarities with the US DMF, EU ASMF, and CEP. However, there are gaps in requirements, including compliance with Chinese Pharmacopoeia (CHP), specific testing data, and others that must be addressed for successful DMF registration in China.
For more on China DMF, click here for our DMF guide.
Videos

China Drug Master File (DMF) Comparing with EU & US
DMF in China, EU and US
How to navigate the China DMF Filing Process
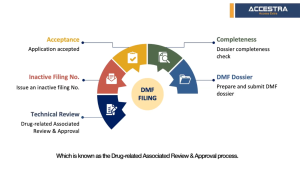
Check material completeness to identify if the materials are generally complete.
Conduct a more detailed data-gap analysis, providing guidance on supplementing missing data according to China CDE requirenents.
Prepare a DMF dossier in Chinese and file to CDE.
CDE reviews dossier for completeness and feedback in 1 to 2 weeks.
CDE issues the Inactive filing number in around 3-6 months counting from step 2 data gap analysis.
CDE performs technical review. The queuing time at CDE may differ depending on the actual situation. It was slightly longer due to Covid-19 but now recovered back to normal processing times.
- Application Form
- Administration Documents
- 2.3.S&3.2.S (ICH M4 CTD)
- Characterisation
- Process Validation
- Impurity Research
- Specification
- CoAs
- Container Closure System
- Stability Study
- Application Form
- Applicant Information
- Quality Attributes
- Characterisation
- Manufacturing Process
- Specification
- CoAs
- Stability Test Report
- Pharmacology and Toxicology Study
- Application Form
- Applicant Information
- Quality Attributes
- Manufacturing Process
- Specification
- Medthod·CoAs
- Stability Test Report
- Compatibility Study
- Safety Study